
Current Issue Archive
-
SHI Lingling, FAN Anchuan, LI Jian, ZHANG Ninghao, LI Yuan, XIN Lixue, JIN Zhengyao
Sciences of Conservation and Archaeology. 2024,36(1):1-10
DOI: 10.16334/j.cnki.cn31-1652/k.20231103080The Shiyu No.2 shipwreck site is located atop coral reefs on the east side of Shiyu in the Xisha Islands, and represents a significant underwater cultural relic within the waters of the Xisha Islands. The site has yielded a variety of porcelain wares including blue and white porcelain, egg-white glazed, white glazed, grayish-green glazed and brownish glazed ones, making it a pivotal window into the exploration of the ancient Maritime Silk Road and the export porcelain trade. Although previous researchers established the relative chronology of the site through typological analysis of blue and white porcelains, the absence of the ship’s structural remains precluded radiocarbon dating. Instead, the thermoluminescence dating method provides an alternative method to determine the absolute chronology of the site. To investigate the thermoluminescence properties and chronology of blue and white porcelains from the site, we conducted tests on 16 porcelain shards using the pre-dose saturation exponential method. The contents of radioactive elements U, Th and 40K in the samples were analyzed using inductively coupled plasma-mass spectrometry (ICP-MS) and inductively coupled plasma-optical emission spectrometry (ICP-OES). During the dose rate calculation, key parameters including the sample’s latitude and longitude, burial depth, moisture content and contents of radioactive elements were considered. The TESCAN integrated mineral analyzer (TIMA) was utilized for the first time to determine the distribution of quartz grain sizes in the porcelain body, thereby enhancing the accuracy of the dose rate estimation. The thermoluminescence dating results indicate that this batch of blue and white porcelains was fired in a period dating from the Yuan Dynasty to the early Ming Dynasty. Within the limited error range of thermoluminescence, this is consistent with the conclusion drawn from traditional appraisal methods that the site belongs to the Yuan Dynasty. This contributes to revealing the manufacturing techniques, practical uses and possible origins of these artifacts from that historical period. Furthermore, it provides important research clues for understanding the communication routes, trade scale and cultural impact of the blue and white porcelain in maritime trade during this era.Citation
Export BibTex EndNote -
LU Xiaoke, ZONG Ruofei, LI Weidong, XU Changsong
Sciences of Conservation and Archaeology. 2024,36(1):11-20
DOI: 10.16334/j.cnki.cn31-1652/k.20230802976The emergence of white porcelain represents a crucial breakthrough in the history of Chinese ceramics, laying a technological foundation for the subsequent prosperity of blue-and-white and painted porcelains. It is widely regarded as the “fourth milestone” in the history of Chinese ceramic science and technology. Therefore, the origination of white porcelain is a pressing issue in the fields of archaeology and archaeometry, which focuses on the remains of the Northern Dynasties and Sui Dynasty from the Xing kiln site excavated in 2012. In this study, inductively coupled plasma-mass spectrometry (ICP-MS) and thermoelectric ionization mass spectrometry (TIMS) were used to analyze the raw materials for early Xing wares from the Fuwulou kiln site in Neiqiu, providing scientific evidence as to the evolution of early porcelain technology at Xing kiln. The results show that the early Xing ware bodies can be divided into two categories based on the trace element pattern, indicating that two kinds of raw materials were used for making porcelain. It further shows that the Xing potters intentionally selected a higher-quality raw material distinct from that of celadon for the production of new wares. The early white porcelain contains generally lower levels of trace elements such as V, Cr, Ni, Nb, Ta, Zr, and Hf, which are closely related to the impurities of zircon and rutile. The strontium (Sr) isotopic mixing pattern analysis demonstrates that the glaze-making for early Xing ware did not generally follow the recipe of wood ash mixed with body clay, but rather that it was mixed with other glaze-making clays. Furthermore, there are discrepancies in strontium isotopic compositions between the early white porcelain and celadon glaze, suggesting that Xing potters attempted to improve the whiteness by modifying the glaze-making technology. These findings scientifically reveal the raw material characteristics and sources of early Xing wares and provide valuable insights into the origination of white porcelain.Citation
Export BibTex EndNote -
LIU Wei, WU Na, CHENG Xiaoxiang
Sciences of Conservation and Archaeology. 2024,36(1):21-31
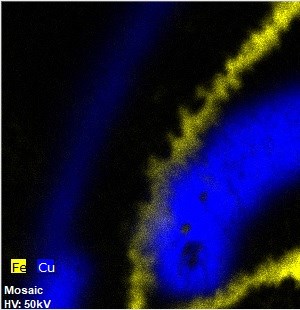
DOI: 10.16334/j. cnki.cn31-1652/k.20231103087The stability of corrosion products on iron artifacts has significant effects on the preservation and conservation of the objects. This is a crucial factor in evaluating the overall conservation state of iron objects. The protective ability index (PAI), which primarily considers the ratio of stable phase to unstable phases, is applied to assess the protective ability of the corrosion layer and the corrosion tendency of iron objects. Recently, Raman spectrometry has been more often used as a semi-quantitative approach for quantifying various components of iron corrosion, making it a valuable tool for calculating the PAI value. In this study, Raman spectrometry, combined with macro X-ray florescence imaging and other techniques, was applied to quantify the composition of corrosion products on an iron bar excavated from the Nanhai No.1 shipwreck. Subsequently, the PAI values were calculated. The results indicate that the corrosion products predominantly comprise four types:α-FeOOH, γ-FeOOH, β-FeOOH and Fe3O4. These products present distinct distribution patterns in the inner and outer layers of the object. The PAI values ranged primarily from 1 to 10, with the lowest recorded at 0.34 and highest at 20.38. Notably, the PAI values for corrosion in outer layer are higher than those in the inner layer, suggesting greater stability in the outer rust. Corrosion present at the stripping surface, with a PAI value below 1, is indicative of significant instability. Similarly, instances of unstable corrosion were observed within the inner layer, as evidenced by a PAI value of 0.73. The application of PAI values in assessing iron corrosion provides a quantitative and reliable method for diagnosing the conservation state of iron objects.Citation
Export BibTex EndNote -
WU Huimin, WANG Yuchen, LIU Siran, HUA Chunyong, LIU Tian, SUN Ao
Sciences of Conservation and Archaeology. 2024,36(1):32-43
DOI: 10.16334/j.cnki.cn31-1652/k.20231103057Huangkuangchang site in Dali, Yunnan Province is a large lead and silver production site of the Ming and Qing Dynasties. In previous studies, the mining age and total amount of silver tax at the site have been discussed, but further research is needed on the smelting technology used. This study reconstructed the lead and silver smelting technology of Huangkuangchang site by analyzing the smelting slags at four locations of this site. The results show that the slags from the various locations at Huangkuangchang site can be categorized into four groups—A, B, C and D, based on the differences in their morphological characteristics, major and trace elements, rare-earth element partition curves, and lead isotopic ratios. A, B and C slags were smelted using roasting-smelting, while the D slag was smelted using the iron reduction process. The lead isotopic analysis results show similar lead isotopic signatures for the ores corresponding to B and C slags, while the D slag has significantly higher lead isotope ratios. The analysis results of rare earth elements show that δEu of the B slag is negatively anomalous, while those of the other groups are slightly positively anomalous. For the A slag, one piece contains highly radioactive lead but the rest are similar to B and C slags, and the trace element content is more discrete while the distribution range is much larger than those of other groups, showing a more diversified source of silver-lead minerals. In summary, workers at Huangkuangchang site may have used a variety of processes for smelting, and lead minerals used may have come from a variety of different deposits, suggesting that the site underwent a number of changes in production techniques and resources over its long production period.Citation
Export BibTex EndNote
Current Issue

Editor-in-Chief:YANG Zhigang
Sciences of Conservation and Archaeology
Volume 36,2024 Issue 1
- LinksShanghai Museum
-
 WeChat ID
WeChat ID -
 Mobile Terminal
Mobile Terminal
Sciences of Conservation and Archaeology ® 2024 All Rights Reserved


 Abstract
Abstract HTML
HTML [PDF 3.37 M]
[PDF 3.37 M] Get Citation
Get Citation